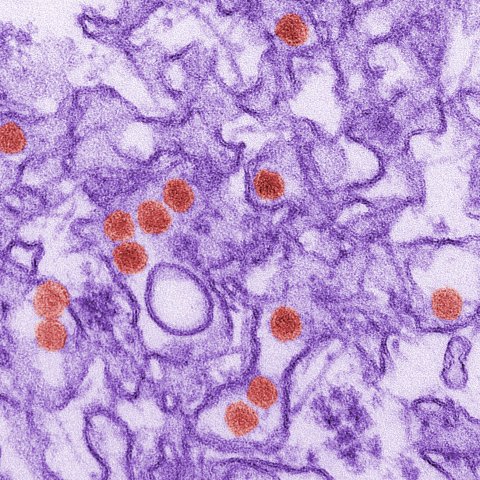
RNA Institute Study Explores Cellular Response to Zika Virus Infection

By Erin Frick
ALBANY, N.Y. (Oct. 31, 2024) — Developing new drugs to treat and prevent viral infections requires a precise understanding of how the virus takes hold and how the host cells respond. In a new study published in the Journal of Virology, University at Albany researchers investigated how the human body fights off Zika virus at the cellular level. Their findings illuminate new potential intervention points for therapeutics against Zika, as well as other RNA viruses.
The researchers focused on a protein called ATF3 (Activating Transcription Factor 3), which plays a critical role in managing cell stress and immune responses during a Zika infection. They also explored a chain of cellular processes called the “integrated stress response” pathway which is instrumental for cells to overcome a variety of stresses.
"Our study provides new insights into how the cell regulates gene expression during Zika virus infection," said lead author Pheonah Badu, who completed the research as a graduate student in UAlbany’s Biology Department and is now a postdoctoral fellow at Regeneron Pharmaceuticals. "For the first time, we discovered that Zika virus activates ATF3 through a network of cellular pathways known as the integrated stress response.
"By identifying the pathway that activates ATF3 during Zika infection and showing how ATF3 enhances the antiviral response, our study links virus-induced stress responses with the regulation of the body's defense mechanisms. These findings enhance our understanding of how viruses affect multiple cellular pathways and could guide future therapeutic approaches."
Zika is primarily spread by mosquitoes. It can also be transmitted sexually or from mother to fetus, resulting in birth defects such as microcephaly and other neurological problems. Guillain-Barré syndrome, a condition that causes muscle weakness and paralysis, is another Zika-induced complication. Currently, there is no antiviral treatment or vaccine.
"When designing a medication or vaccine against a virus, you could target the virus itself, but because many viruses can mutate and develop resistance, there is a chance the medication or treatment could become ineffective," said coauthor Cara Pager, associate professor in the Department of Biological Sciences and the RNA Institute.
"Another approach is targeting a process in the host cell that the virus needs to replicate. By turning off a function that the virus needs, it won’t be able to survive. However, if that process is something that the host also requires, then turning it off could be detrimental to both the virus and the host. Exploring these push-pull interactions between the host and virus, like we’ve done in this study, will help develop effective treatments."
Studying Zika infection at the cellular level
RNA viruses like Zika infect human cells and then take over the endoplasmic reticulum—a cellular structure involved in producing proteins.
"Lacking their own machinery to replicate, viruses must co-opt processes in the host cell in order to survive and multiply," said coauthor Morgan Sammons, associate professor in the Department of Biological Sciences and the RNA Institute. "This triggers a cellular defense mechanism wherein stress-related proteins like ATF3 are activated to help restore the cell to a stable state."
To observe how Zika virus affects ATF3, the team infected human cells, specifically a lung cancer cell line, with the virus. Using CRISPR gene editing, they removed the ATF3 gene in some cells to compare levels of ATF3 and other Zika-related proteins in the infected versus normal cells. They also used inhibitors to block specific stress response pathways, to see how preventing stress responses affects Zika virus replication.
Identifying druggable intervention points
The team found that Zika virus infection triggers the "integrated stress response" pathway, a series of cellular stress responses which induce ATF3 activity. In turn, ATF3 activates antiviral genes and other immune factors that work together against the virus. These findings demonstrate "crosstalk" between infected cells’ stress response and immune response pathways, highlighting the importance of ATF3 in activating the immune system and limiting viral replication.
"In order to develop new treatments and vaccines against RNA viruses, we need to understand what our cells and tissues do when they encounter them," said Sammons. "We know there are multiple different ways that cells respond to RNA viruses, including turning on danger signals that incite stress response pathways that help mediate the virus.
"In the case of Zika, we understand how the virus uses the host cell’s machinery. We also need to understand how the cell is counteracting that attack. Identifying the mechanisms that drive these response pathways offers new potential intervention points, which could mean more ways to stop the virus."
The research team credits the structure of UAlbany’s Life Sciences Research Initiative and the RNA Institute for bringing their work together. While Pager and Sammons typically pursue very different lines of research, the proximity of their labs opened a new creative pathway.
"One of the major strengths of this work is that we combined the expertise of two research groups from the biology department: virologists Cara Pager and Pheonah Badu and genomic scientists Morgan Sammons and myself," said coauthor Gabriele Baniulyte, research scientist in the Sammons Lab. "This dual-perspective approach allowed us to dissect the molecular basis of the stress response in human cells after Zika virus infection. This made for a fantastic collaboration that provided a snapshot of changes in gene expression in response to viral infection, and how ATF3 fits in to remediate this type of stress."